How Many Atoms Are Needed To Form A Molecule
12.2: The Mole
- Page ID
- 199964
Learning Objectives
- To define the mole unit.
Figure \(\PageIndex{ane}\) shows that nosotros need 2 hydrogen atoms and 1 oxygen atom to make 1 water molecule. If nosotros want to make 2 h2o molecules, nosotros volition need 4 hydrogen atoms and 2 oxygen atoms. If we want to make 5 molecules of water, nosotros need ten hydrogen atoms and 5 oxygen atoms. The ratio of atoms we volition demand to make whatever number of water molecules is the same: 2 hydrogen atoms to 1 oxygen atom.

One problem we accept, notwithstanding, is that information technology is extremely difficult, if not impossible, to organize atoms i at a time. As stated in the introduction, nosotros deal with billions of atoms at a time. How tin nosotros proceed track of then many atoms (and molecules) at a time? We do it by using mass rather than by counting individual atoms.
A hydrogen atom has a mass of approximately ane u. An oxygen atom has a mass of approximately xvi u. The ratio of the mass of an oxygen atom to the mass of a hydrogen cantlet is therefore approximately xvi:1.
If we have ii atoms of each chemical element, the ratio of their masses is approximately 32:2, which reduces to 16:i—the same ratio. If we have 12 atoms of each element, the ratio of their total masses is approximately (12 × 16):(12 × 1), or 192:12, which also reduces to 16:i. If nosotros have 100 atoms of each element, the ratio of the masses is approximately 1,600:100, which again reduces to sixteen:1. Equally long every bit we have equal numbers of hydrogen and oxygen atoms, the ratio of the masses will always be 16:1.
The same consistency is seen when ratios of the masses of other elements are compared. For instance, the ratio of the masses of silicon atoms to equal numbers of hydrogen atoms is always approximately 28:1, while the ratio of the masses of calcium atoms to equal numbers of lithium atoms is approximately 40:seven.
And so we have established that the masses of atoms are abiding with respect to each other, equally long as nosotros have the same number of each type of cantlet. Consider a more macroscopic example. If a sample contains 40 grand of Ca, this sample has the same number of atoms as at that place are in a sample of 7 thousand of Li. What we need, and then, is a number that represents a convenient quantity of atoms so nosotros can relate macroscopic quantities of substances. Conspicuously even 12 atoms are likewise few because atoms themselves are so small. Nosotros need a number that represents billions and billions of atoms.
Chemists utilize the term mole to represent a large number of atoms or molecules. Just as a dozen implies 12 things, a mole (mol) represents 6.022 × 1023 things. The number 6.022 × 1023, called Avogadro's number later the 19th-century chemist Amedeo Avogadro, is the number we utilise in chemical science to represent macroscopic amounts of atoms and molecules. Thus, if we have six.022 × 1023 O atoms, we say we have ane mol of O atoms. If we take 2 mol of Na atoms, nosotros have 2 × (half dozen.022 × 1023) Na atoms, or 1.2044 × 1024 Na atoms. Similarly, if we have 0.5 mol of benzene (C6Hhalf dozen) molecules, we have 0.5 × (6.022 × 1023) CviHhalf-dozen molecules, or iii.011 × 1023 C6Hhalf-dozen molecules.
A mole represents a very large number! If 1 mol of quarters were stacked in a cavalcade, it could stretch back and forth between Earth and the sun 6.8 billion times.
Notice that we are applying the mole unit to unlike types of chemic entities. In these examples, we cited moles of atoms and moles of molecules. The word mole represents a number of things—6.022 × 1023 of them—but does not by itself specify what "they" are. They can be atoms, formula units (of ionic compounds), or molecules. That information still needs to exist specified.
Because 1 Hii molecule contains ii H atoms, one mol of H2 molecules (6.022 × 1023 molecules) has 2 mol of H atoms. Using formulas to indicate how many atoms of each element we have in a substance, nosotros tin chronicle the number of moles of molecules to the number of moles of atoms. For example, in ane mol of ethanol (C2Hhalf dozenO), we can construct the following relationships (Table \(\PageIndex{1}\)):
| 1 Molecule of \(C_2H_6O\) Has | ane Mol of \(C_2H_6O\) Has | Molecular Relationships |
|---|---|---|
| two C atoms | ii mol of C atoms | \(\mathrm{\dfrac{2\: mol\: C\: atoms}{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{ane\: mol\: C_2H_6O\: molecules}{2\: mol\: C\: atoms}}\) |
| 6 H atoms | 6 mol of H atoms | \(\mathrm{\dfrac{6\: mol\: H\: atoms}{ane\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{half dozen\: mol\: H\: atoms}}\) |
| 1 O atom | 1 mol of O atoms | \(\mathrm{\dfrac{1\: mol\: O\: atoms}{ane\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{1\: mol\: O\: atoms}}\) |
The post-obit example illustrates how we can use these relationships every bit conversion factors.
Example \(\PageIndex{1}\)
If a sample consists of 2.5 mol of ethanol (C2HviO), how many moles of carbon atoms, hydrogen atoms, and oxygen atoms does it have?
SOLUTION
Using the relationships in Tabular array \(\PageIndex{1}\), we utilise the appropriate conversion gene for each element:
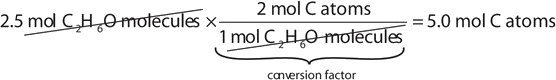
Notation how the unit of measurement mol C 2 H 6 O molecules cancels algebraically. Like equations tin can exist synthetic for determining the number of H and O atoms:
\(\mathrm{ii.five\: mol\: C_2H_6O\: molecules\times\dfrac{6\: mol\: H\: atoms}{1\: mol\: C_2H_6O\: molecules}=15\: mol\: H\: atoms}\)
\(\mathrm{2.5\: mol\: C_2H_6O\: molecules\times\dfrac{one\: mol\: O\: atoms}{1\: mol\: C_2H_6O\: molecules}=2.5\: mol\: O\: atoms}\)
Exercise \(\PageIndex{ane}\)
If a sample contains six.75 mol of Na2So4, how many moles of sodium atoms, sulfur atoms, and oxygen atoms does it accept?
The fact that 1 mol equals 6.022 × 1023 items tin can likewise exist used every bit a conversion cistron.
Case \(\PageIndex{2}\)
How many formula units are nowadays in 2.34 mol of NaCl? How many ions are in 2.34 mol?
SOLUTION
Typically in a problem like this, we showtime with what nosotros are given and apply the appropriate conversion factor. Here, nosotros are given a quantity of 2.34 mol of NaCl, to which we can apply the definition of a mole equally a conversion factor:
\(\mathrm{two.34\: mol\: NaCl\times\dfrac{half-dozen.022\times10^{23}\: NaCl\: units}{ane\: mol\: NaCl}=1.41\times10^{24}\: NaCl\: units}\)
Because there are 2 ions per formula unit, there are
\(\mathrm{1.41\times10^{24}\: NaCl\: units\times\dfrac{ii\: ions}{NaCl\: units}=2.82\times10^{24}\: ions}\)
in the sample.
Exercise \(\PageIndex{two}\)
How many molecules are nowadays in 16.02 mol of C4Hten? How many atoms are in sixteen.02 mol?
Concept Review Exercise
-
What is a mole?
Answer
-
A mole is 6.022 × 1023 things.
Key Takeaway
- A mole is 6.022 × x23 things.
Exercises
-
How many dozens are in 1 mol? Express your answer in proper scientific notation.
-
A gross is a dozen dozen, or 144 things. How many gross are in one mol? Express your answer in proper scientific notation.
-
How many moles of each blazon of cantlet are in 1.0 mol of CviH12O6?
-
How many moles of each blazon of cantlet are in i.0 mol of GrandtwoCr2Ovii?
-
How many moles of each type of atom are in 2.58 mol of NatwoSo4?
-
How many moles of each type of atom are in 0.683 mol of C34H32FeN4O4? (This is the formula of heme, a component of hemoglobin.)
-
How many molecules are in sixteen.eight mol of H2O?
-
How many formula units are in 0.778 mol of atomic number 26(3) nitrate?
-
A sample of gold contains 7.02 × 1024 atoms. How many moles of aureate is this?
-
A flask of mercury contains 3.77 × 1022 atoms. How many moles of mercury are in the flask?
-
An intravenous solution of normal saline may contain i.72 mol of sodium chloride (NaCl). How many sodium and chlorine atoms are present in the solution?
-
A lethal dose of arsenic is one.00 × 1021 atoms. How many moles of arsenic is this?
Answers
-
v.018 × ten22 dozens
-
half dozen.0 mol of C atoms, 12.0 mol of H atoms, and six.0 mol of O atoms
-
five.16 mol of Na atoms, 2.58 mol of South atoms, and 10.32 mol of O atoms
-
1.012 × 1025 molecules
-
11.7 mol
-
1.04 × 1024 Na atoms and 1.04 × ten24 Cl atoms
Source: https://chem.libretexts.org/Courses/Modesto_Junior_College/Chemistry_150_-_Bunag/Textbook_for_Chemistry_150/12%3A_Quantities_in_Chemical_Reactions/12.02%3A_The_Mole

0 Response to "How Many Atoms Are Needed To Form A Molecule"
Post a Comment